More Kinetics Links
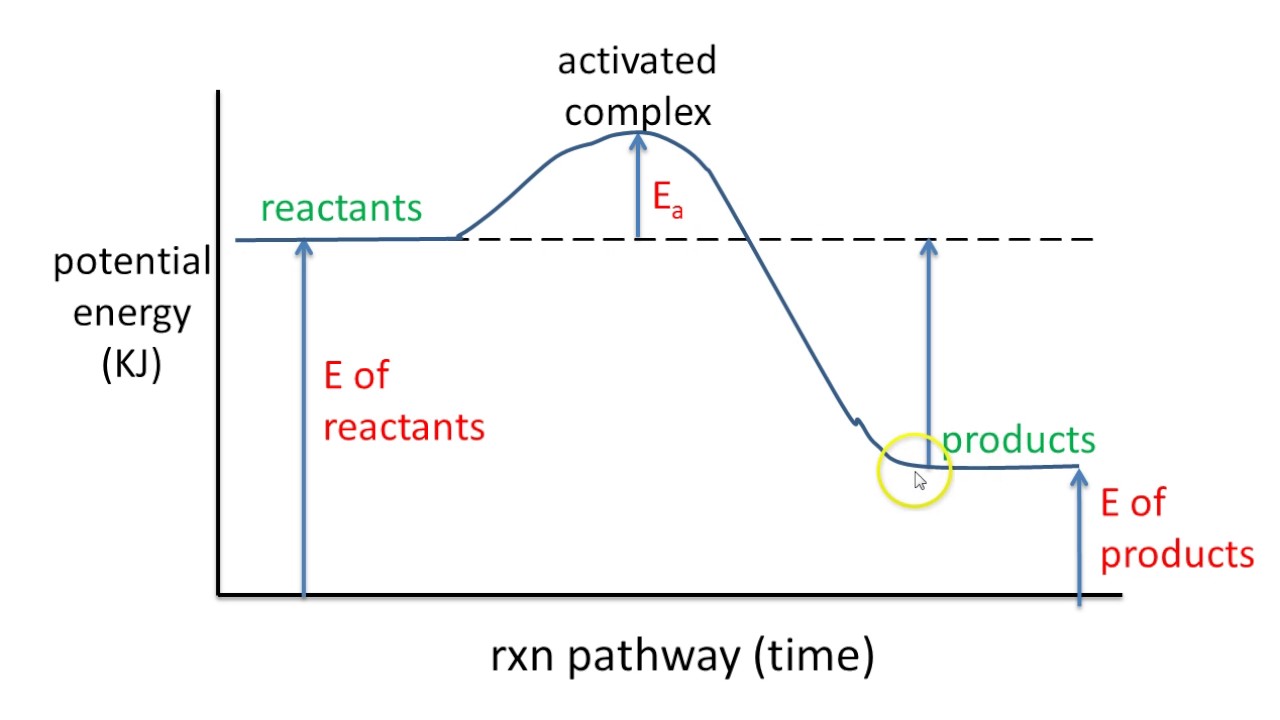
A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram. Sometimes a teacher finds it necessary to ask questions about PE diagrams that involve actual Potential Energy values. This short video takes you through a few example of those problems.
More on PE Diagrams ENERGY FLOW
Activation Energy 'Ea' -The Energy required to initiate a chemical reaction. Both endothermic and exothermic reactions require activation energy.
Activated complex
Effect of a Catalyst speeds up a reaction by lower the activation energy More Kinetics Links Regents Questions-Highlight to reveal answer 6/02 Base your answers on the information and diagram below, which represent the changes in potential energy that occur during the given reaction. Given the reaction: A + B --> C a) Does the diagram illustrate an exothermic or an endothermic reaction? State one reason, in terms of energy, to support your answer.
b) On the diagram provided in your answer booklet,draw a dashed line to indicate a potential energy curve for the reaction if a catalyst is added.
8/02 1. Given the reaction: 2 H2(g) + O2(g) --> 2 H2O(l) + 571.6 kJ What is the approximate ΔH for the formation of 1 mole of H2O(l)? (1) -285.8 kJ (2) +285.8 kJ (3) -571.6 kJ (4) +571.6 kJ
2. According to Table I, which potential energy diagram best represents the reaction that forms H2O(l) from its elements?
3. Given the reaction: CH4(g) + 2 O2(g) --> 2 H2O(l) + CO2(g) What is the overall result when CH4(g) burns according to this reaction? (1) Energy is absorbed and ΔH is negative. (3) Energy is released and ΔH is negative. (2) Energy is absorbed and ΔH is positive. (4) Energy is released and ΔH is positive.
4. According to Table I, which salt releases energy as it dissolves? (1) KNO3 (2) LiBr (3) NH4NO3 (4) NaCl
1/03 Given the reaction: S(s) + O2(g) à SO2(g) + energy Which diagram best represents the potential energy changes for this reaction?
6/03 22. Which statement correctly describes an endothermic chemical reaction? (1) The products have higher potential energy than the reactants, and the ΔH is negative. (2) The products have higher potential energy than the reactants, and the ΔH is positive. (3) The products have lower potential energy than the reactants, and the ΔH is negative. (4) The products have lower potential energy than the reactants, and the ΔH is positive.
35. A catalyst is added to a system at equilibrium. If the temperature remains constant, the activation energy of the forward reaction (1) decreases (2). increases (3) remains the same
43. The potential energy diagram below represents a reaction. Which arrow represents the activation energy of the forward reaction? (1) A (2) B (3) C (4) D
8/03 17 Which phase change is an exothermic process? (1) CO2 (s) --> CO2 (g) (2) NH3 (g) --> NH3 (l) (3) Cu(s) --> Cu(l) (4) Hg(l) --> Hg(g)
51 Explain how a catalyst may increase the rate of a chemical reaction.
52 Sketch the potential energy diagram for an endothermic chemical reaction that shows the activation energy and the potential energy of the reactants and the potential energy of the products.
1/04 16 Which statement best explains the role of a catalyst in a chemical reaction? (3) A catalyst changes the kinds of products produced. (4) A catalyst provides an alternate reaction path-way that requires less activation energy.
Base your answers to questions 77 through 79 on the information and potential energy diagram below. Chemical cold packs are often used to reduce swelling after an athletic injury. The diagram represents the potential energy changes when a cold pack is activated. 77 Which lettered interval on the diagram represents the potential energy of the products?
More Kinetics Links 6/04 21 A catalyst increases the rate of a chemical reaction by
8/04 19 Which information about a chemical reaction is provided by a potential energy diagram? (1) the oxidation states of the reactants and products (2) the average kinetic energy of the reactants and products (3) the change in solubility of the reacting substances (4) the energy released or absorbed during the reaction
1/05 Base your answers to questions 56 through 58 on the potential energy diagram and the equation below. 2 C(s) + H2(g) + 227.4 kJ → C2H2(g) 56 The letter B represents which chemical formula or formulas in the equation?
57 If 682.2 kilojoules are absorbed, how many moles of C2H2(g) are produced?
58 Describe how the potential energy diagram will change if a catalyst is added.
1/06 16 In a chemical reaction, the difference between the potential energy of the products and the potential energy of the reactants is defined as the (1) activation energy (2) ionization energy (3) heat of reaction (4) heat of vaporization
40 Given the balanced equation:
43 Given the potential energy diagram for a chemical reaction: Which statement correctly describes the energy changes that occur in the forward reaction? (1) The activation energy is 10. kJ and the reaction is endothermic. (2) The activation energy is 10. kJ and the reaction is exothermic. (3) The activation energy is 50. kJ and the reaction is endothermic. (4) The activation energy is 50. kJ and the reaction is exothermic.
6/06 18 Which expression represents the ΔH for a chemical reaction in terms of the potential energy, PE, of its products and reactants? (1) PE of products + PE of reactants (2) PE of products – PE of reactants (3) PE of products × PE of reactants (4) PE of products ÷ PE of reactants
19 Which balanced equation represents an endothermic reaction?
Base your answers to questions 59 and 60 on the information below. Given the reaction at equilibrium: 59 Explain, in terms of energy, why the forward reaction is exothermic.
60 Explain, in terms of Le Chatelier’s principle, why the equilibrium shifts to the right to relieve the stress when the pressure on the system is increased at constant temperature.
8/06 16 Which statement best describes how a catalyst increases the rate of a reaction? (1) The catalyst provides an alternate reaction pathway with a higher activation energy. (2) The catalyst provides an alternate reaction pathway with a lower activation energy. (3) The catalyst provides the same reaction pathway with a higher activation energy. (4) The catalyst provides the same reaction pathway with a lower activation energy.
44 Given the balanced equation representing a reaction: Which statement is true about energy in this reaction? (1) The reaction is exothermic because it releases heat. (2) The reaction is exothermic because it absorbs heat. (3) The reaction is endothermic because it releases heat. (4) The reaction is endothermic because it absorbs heat.
46 Given the potential energy diagram for a reaction: Which interval on this diagram represents the difference between the potential energy of the products and the potential energy of the reactants? (1) 1 (2) 2 (3) 3 (4) 4 More Kinetics Links Chemical Demonstration Videos |
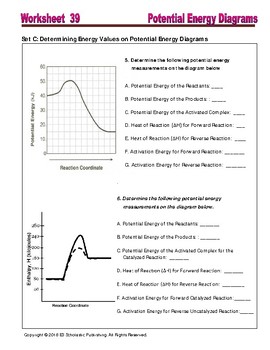

Potential Energy Diagram Worksheet 1. The heat content of the reactants of the forward reaction is about kilojoules. The heat content of the products of the forward reaction is about kilojoules. Try these worksheets for middle, high school, and AP students on motion, energy, forces, simple machines, magnets, electromagnetic spectrum, and more. Take a quiz to test knowledge of electrical.
Potential Energy Diagram Worksheets


Potential Energy Diagram Worksheet Chemistry
Find the latest printable worksheets and activities for teachers, parents, tutors and homeschool families on fiat.aluccia.com. 6 forms of energy 7 forms of energy forms of energy worksheet types of energy sources Forms of Energy 9th class physics Chapter 6 Work and Energy online lecture. Kinetic and Potential Energy Worksheet - Kinetic and Potential Energy Worksheet, Potential or Kinetic Energy Worksheet Stem Energy.